Why this guide?
In recent years cannabis trade and related activity has developed into a growth business area. In the life sciences and healthcare fields, there have been increases in the numbers of patients having access to medicinal cannabis and more general acceptance of the potential medicinal value of cannabis for patients. The number of countries allowing its recreational use has increased. Additionally, there is growing awareness of the potential for, and actual use of, cannabis plants across a range of different industry sectors, not least in cosmetics and food.
Taking account of the evolving political and ethical implications of cannabis production, trade and use, it is important to be aware of differences in approach across different countries to assess which businesses and activities are viable in different (EU and non-EU) jurisdictions.
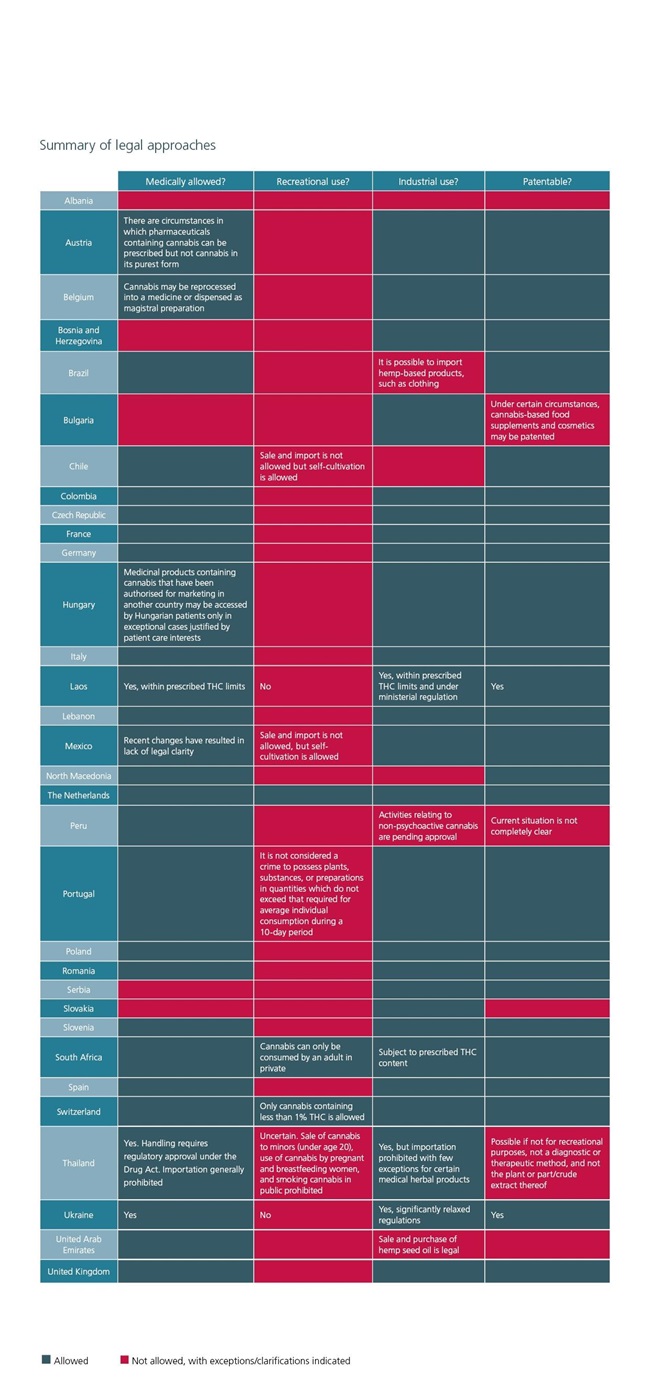
This guide provides high level information about the legal approach to regulating cannabis across a variety of jurisdictions in different regions. In each case the guide focuses on
- medical use;
- recreational use;
- industrial use; and
- the patentability of cannabis-based products.
Key concepts
Cannabis plant
The cannabis plant has a wide range of industrial and medical applications. The hemp strain is used in building materials, textiles, paint and biofuel, to name a few examples, while other cannabis varieties are grown for their cannabinoid profiles. Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the best-known cannabinoids, but there are more than 100 other compounds in this class. This huge versatility has led to a burgeoning industry centred around the cannabis plant.
Medical use
The medical use of cannabis exploits the action of cannabinoids synthesised by the cannabis plant. Conditions that are treated with cannabis-derived products include chronic pain, pain associated with multiple sclerosis and spinal cord injury, nausea and vomiting caused by chemotherapy, radiotherapy and HIV therapies, appetite stimulation in cachexia and anorexia, loss of appetite in cancer patients and those with AIDS or anorexia nervosa, the hypotensive effect of glaucoma and the reduction of involuntary body and facial movements in Gilles de la Tourette syndrome.
Medical use is widespread around the world, with some notable exceptions. Obtaining authorisation is generally necessary to be able to produce, import and sell cannabis for medical use. Patients generally gain access to these products via a prescription.
Industrial use
Before discovering the healing properties of cannabis, its use was mainly connected to industry. Although some countries (especially in Europe) have adopted a distinction between the varieties of cannabis that, generally being used in the industrial sector, do not fall within the scope of laws on psychotropic substances, not all countries have made this distinction.
In the European Union (EU), the cultivation of industrial cannabis (hemp) is permitted provided the variety is registered in the EU’s ‘Common Catalogue of Varieties of Agricultural Plant Species‘ and the THC content does not exceed 0.2%. This measure has been taken with the aim of encouraging cultivation.
Industrial cannabis usually contains only traces of THC, while CBD is present. Not all countries have clear regulation on CBD.
Patentability
Cannabis-based products are potentially patentable in most territories. The normal principles of patentability generally apply to these products, without ad hoc legislation.
Summary of legal approaches
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.
Source Mondaq https://www.mondaq.com/cannabis–hemp/1452986/cms-expert-guide-to-cannabis-law-and-legislation





















