Medicinal cannabis, CBD and hemp healthcare products company, Bod Australia Limited (ASX:BDA) has provided its shareholders an update for the Q3FY2021 quarter, ended 31 March 2021.
Significant progress was made by BDA during the quarter, with growth underpinned by strong MediCabilisTM prescription sales, ongoing R&D initiatives to build real world data supporting the efficacy of the Company’s medicinal cannabis products on a range of debilitating conditions and the appointment of a new executive.

CEO Jo Patterson commented on these updates: “The increase in total invoiced sales for the period is very pleasing and growth can be attributed to the completion of a number of binding purchase orders from H&H, which will result in Bod’s products being sold in key markets across Europe and the UK.
“The continued upward trajectory in medicinal cannabis sales throughout Australia has also been a major factor in building our revenue profile.
“We expect additional purchase orders to support our US market entry to be secured during the quarter, as well as the launch of our products to European consumers.
“We also anticipate that medicinal cannabis sales will continue to track upward in line with the growing number of users in Australia.
Growth in MediCabilisTM sales will also be underpinned by uptake in the UK and the Company’s ability to execute on strategic R&D initiatives.
“The Company remains in a strong financial position with over $9.2m in cash at bank and this leaves us very well placed to pursue a number of growth objectives.”
Growth in medical cannabis sales
An upward trajectory in MediCabilisTM prescriptions has added to BDA’s growing revenue profile and the company expects uptake to continue over the coming quarters in both Australia and the UK.
Indeed, BDA filled a total of 3,789 MediCabilisTM prescriptions during Q3 FY2021, highlighting a 61% increase on the previous quarter.
Volumes during the quarter also take the told number of MediCabilisTM units sold during FY2021 to 7,730, representing a 93% increase on total FY2020 volumes.
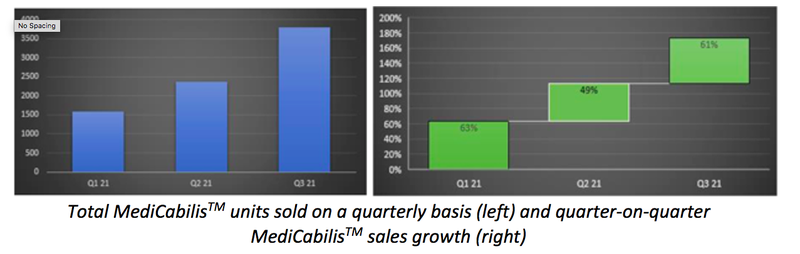
Prescription volume growth has been underpinned by BDA’s established relationships with approved prescribers, educational initiatives with physicians and the Company’s ongoing nationwide trial to test the efficacy of MediCabilisTM on conditions including anxiety, insomnia and Post Traumatic Stress Disorder (PTSD).
High level of patient and physician satisfaction for Bod’s cannabis products in the Australian market is represented by 62% of MediCabilisTM prescriptions filled during Q3 FY2021 were repeat prescriptions.
Corporate overview
BDA’s total invoiced sales for the period were $2,744,000 a 188% increase on the previous quarter ($0.95m).
Growth in total invoiced sales is due to the delivery of Bod’s CBD and hemp products to key markets including France, the Netherlands, Italy in the UK following binding purchase orders from exclusive global partner Health and Happiness Group Limited (H&H).
Sales of Bod’s MediCabilisTM product also added to revenue. At the end of the quarter, BDA also had unfulfilled binding purchase orders on hand valued at $0.35m
BDA retained a strong cash balance of over $9.2m at the end of the quarter. This provides the Company with considerable financial flexibility to pursue a number of near term opportunities.
Collaboration agreement to explore medicinal cannabis efficacy on long-COVID-19
BDA entered into a collaboration agreement with the UK’s leading independent scientific body on drugs, Drug Science UK, to assess the efficacy MediCabilisTM in managing symptoms associated with the long term impact of SARS-CoV-2, which is commonly referred to as long-COVID.
Long-COVID refers to when people experience symptoms of COVID-19 and do no fully recover for several weeks or months after the start of symptoms. The more common symptoms of the condition include sleep disturbance, chronic pain, anxiety and fatigue, all of which may be amenable to treatment with cannabis-based medicinal products.
There are currently no treatments for symptoms associated with long-COVID and a growing need to identify potential ways to manage the clinical condition.
As BDA’s MediCabilisTM product is currently used to treat a range of conditions, including pain and anxiety, which gives the Company confidence that it may assist with long-COVID sufferers.
The two companies will progress a UK-based clinical study to explore the effectiveness of prescribing MediCabilisTM to long-COVID sufferers. Clinical trial protocol is currently being finalised and expected to commence in the coming months.
Bod anticipates that the trial will build on the growing body of evidence for the use of MediCabilisTM, broaden visibility in the UK market and strengthen the Company’s relationship with Drug Science.
Outlook for this quarter and beyond
BDA is focused on a number of revenue generating objectives and growth initiatives during this quarter and beyond, including:
- Progress US market entry with H&H following receipt of initial binding purchase order;
- International market and product expansion with H&H under new and existing brands;
- Continued growth of MediCabilisTM prescription sales in Australia, as well as scale up of UK operations;
- R&D initiatives to further build on the growing body of evidence for the use of MediCabilisTM; and
- Finalise product registration strategy for new schedule 3 CBD products in the Australian market.
https://finfeed.com/small-caps/biotech/bod-australia-outlines-strong-revenue-growth/



















