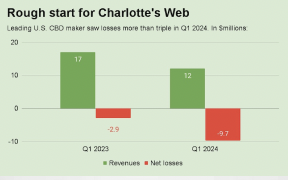
Robert Hoban one of the leading light lawyers in the cannabis & hemp space, based out of Denver CO, has written a state of play report on cannabis & hemp regulations in Canada as we move toward a fully regulated market mid 2018.
He writes, As Canada moves towards legalizing cannabis for adult use, the government is in the process of setting up new regulations for adult-use cannabis, medical cannabis and hemp. These regulations are complicated, and they also will impact Canadian cannabis exports and international investment opportunities.
Of most interest to GHG readers will be the following hemp section
Hemp in Canada
There are many varieties of the cannabis plant, and hemp, also called industrial hemp, refers to the varieties of Cannabis sativa L. which typically contains less than 1 percent tetrahydrocannabinol (or THC). Although cannabis is a controlled substance in Canada, there are exceptions, including medical cannabis use and industrial hemp, under the Industrial Hemp Regulations (IHR).
The IHR defines Industrial Hemp as “the plants and plant parts of the genera Cannabis, the leaves and flowering heads of which do not contain more than 0.3 percent THC”, and permits certain activities with respect to hemp for commercial purposes through a licensing system. However, current licenses granted under the IHR expire in the calendar year they are issued.
Under Canada’s proposed new cannabis regulations, every license or authorization issued under the IHR prior to the commencement date of Bill C-45 will be deemed to be a license issued under Section 62 of that law. According to the draft regulations, non-viable seed, mature stalk without any leaf, flower, seed or branch fall outside the scope of the adult-use law, and therefore no license would be required for processing or sale, or for the sale of any derivatives of seed and grain containing 10 micrograms/grams THC.
The IHR applies to the importation, exportation and possession of industrial hemp and its production, sale, provision, transport, sending or delivering. The IHR does not apply to the importation, exportation, sale or provision of whole industrial hemp plants, including sprouts, or the leaves, flowers or bracts of those plants, or their derivatives, or any derivative of seed, viable or non-viable seed, or products from that derivative, if the derivative or product contain more than 10 micrograms/gram THC.
Both Canada’s Controlled Substances Act and the IHR do not apply to the importation, exportation or wholesale sale of a derivative of seed, viable grain or non-viable cannabis seed, or a product made from that derivative, if not made from whole industrial plants (including sprouts, leaves, flowers or bracts of those plants). The laws also do not apply to an industrial hemp derivative or product that has 10 micrograms/gram THC or less, labelled as such, and accompanied by a certificate of THC from a trusted laboratory. An example of such a derivative or product that the Controlled Substances Act or IHR would not apply to may be hemp seed oil and flour with less 10 micrograms/gram THC or less.
Under the proposed regulations for Canada’s adult-use cannabis market, industrial hemp would be defined as “cannabis plants whose leaves and flowering heads do not contain more than 0.3 percent THC,” and similar to the micro-license framework for non-medical cannabis, industrial hemp licenses will be permitted to possess, transport, research and sell industrial hemp leaves, flowers and branches (or the whole plant).
The regulations will also modify existing physical security requirements for industrial hemp licenses and begin treating industrial hemp storage as any other agricultural product. This includes a proposal to remove a current IHR requirement that industrial hemp be stored in a locked container or location, or premises to which only authorized persons have access.
Currently, industrial hemp licences authorize the cultivation of industrial hemp from approved varieties from pedigreed seeds, those that have been confirmed to consistently produce less than 0.3 percent or less THC. The benefit of using approved pedigreed seeds is that these licensees would be permitted to bypass the lab THC testing requirement. Lab testing would remain mandatory for those Industrial Hemp licensees under the new regulations who do not use pre-approved pedigreed seeds to confirm THC content.
Also included in the article are sections entitled.
- Adult-Use and Medical Cannabis in Canada
- Canadian Capital Markets and Cannabis
- Exporting Canadian Cannabis
Source: https://cannabisnow.com/legal-perspective-inside-canadas-new-cannabis-hemp-regulations/