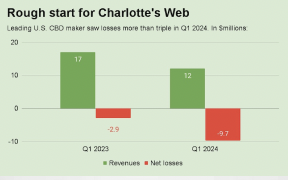
MJ Biz reports…
The French Agency for the Safety of Medicines and Health Products (ANSM) selected the companies that will supply France’s medical cannabis pilot program that’s intended to provide free products to patients enrolled in the trial.
Decision of 25/01/2021 fixing the list of companies selected to provide and distribute free of charge cannabis-based medicines within the framework of the experiment provided for in article 43 of law n ° 2019-1446 of 24 December 2019 social security financing for 2020
01/25/2021
The Director General of the National Agency for the Safety of Medicines and Health Products (ANSM)
Considering article 43 of law n ° 2019-1446 of December 24, 2019 on the financing of social security for 2020 ;
Considering the decree n ° 2020-1230 of October 7, 2020 relating to the experimentation of the medical use of cannabis;
Considering the decree of October 16, 2020 setting the specifications of cannabis-based drugs used during the experimentation provided for in article 43 of law n ° 2019-1446 of December 24, 2019 on the financing of social security for 2020, the conditions of their availability as well as the therapeutic indications or clinical situations in which they will be used;
Considering the publication on October 19, 2020 on the ANSM website, of the call for applications procedure aimed at selecting service providers for the free supply and distribution of cannabis-based drugs for patients who will participate in the ” experimentation with the medical use of cannabis
Considering the closure on November 24, 2020 of the aforementioned call for candidates procedure
Having regard to the various offers presented within the framework of the call for candidates procedure;
Considering the examination by the ANSM of all the application files submitted to it, with regard to the participation and award conditions and the technical response framework published on the ANSM website as well as the requirements defined in the aforementioned decree of October 16 and its appendix relating to the specifications for the free supply and distribution of cannabis-based drugs for patients who will participate in the experimentation of the medical use of cannabis;
Considering the control by the ANSM of the conformity and the quality of the medical cannabis submitted within the framework of the aforementioned application,
Decide:
Article 1 st :
The companies mentioned in article 2 of the abovementioned decree of October 16, 2020, selected to supply and distribute cannabis-based medicines in France during the experimentation of the medical use of cannabis, are exclusively the following, according to of each batch characterized by determined ratios of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD):
Dominant THC ratio
Lot 1.1: Flower THC> 8%, CBD <1%:
-
Name of the main supplier: AURORA EUROPE GmbH in partnership with the pharmaceutical establishment operating L ABORATOIRES ETHYPHARM SAS;
-
Name of the alternate supplier: TILRAY in partnership with the pharmaceutical establishment operating MEDIPHA SANTE.
Lot 1.2: THC oil> 5 mg / ml, CBD <1 mg / ml:
-
Name of the main supplier: TILRAY in partnership with the operating pharmaceutical establishment MEDIPHA SANTE;
-
Name of the alternate supplier: PANAXIA PHARMACEUTICAL INDUSTRIES in partnership with the pharmaceutical establishment operating NEURAXPHARM FRANCE.
Lot 1.3: Oral form to ingest THC> 5 mg / ml, CBD <1 mg / ml:
-
Name of the main supplier: PANAXIA PHARMACEUTICAL INDUSTRIES in partnership with the pharmaceutical establishment operating NEURAXPHARM FRANCE;
-
Name of the alternate supplier: EMMAC LIFE SCIENCES in partnership with the pharmaceutical establishment operating LABORATOIRES BOIRON.
Balanced ratio of THC and CBD
Lot 2.1: Flower THC and CBD> 5%:
-
Name of the main supplier: AURORA EUROPE GmbH in partnership with the pharmaceutical establishment operating LABORATOIRES ETHYPHARM SAS;
-
Name of the alternate supplier: TILRAY in partnership with the pharmaceutical establishment operating MEDIPHA SANTE.
Lot 2.2: THC and CBD oil> 5 mg / ml:
-
Name of the main supplier: TILRAY in partnership with the operating pharmaceutical establishment MEDIPHA SANTE;
-
Name of the alternate supplier: LITTLE GREEN PHARMA in partnership with the pharmaceutical establishment operating INTSEL CHIMOS SAS.
Lot 2.3: Oral form to ingest THC and CBD> 5 mg / ml:
-
Name of the main supplier: PANAXIA PHARMACEUTICAL INDUSTRIES in partnership with the pharmaceutical establishment operating NEURAXPHARM FRANCE;
-
Name of the alternate supplier: EMMAC LIFE SCIENCES in partnership with the pharmaceutical establishment operating LABORATOIRES BOIRON.
Dominant CBD ratio
Lot 3.2: THC oil <1 mg / ml, CBD> 5 mg / ml:
-
Name of the main supplier: LITTLE GREEN PHARMA Ltd in partnership with the pharmaceutical establishment operating INTSEL CHIMOS SAS;
-
Name of the alternate supplier: ALTHEA COMPANY PTY Ltd in partnership with the pharmaceutical establishment operating LABORATOIRES BOUCHARA RECORDATI.
Lot 3.4: Flower THC <5% CBD> 5%:
-
Name of the main supplier: AURORA EUROPE GmbH in partnership with the pharmaceutical establishment operating LABORATOIRES ETHYPHARM SAS;
-
Lack of alternate supplier.
Lot 3.5: THC oil <5 mg / ml, CBD> 5 mg / ml:
-
Name of the main supplier: LITTLE GREEN PHARMA Ltd in partnership with the pharmaceutical establishment operating INTSEL CHIMOS SAS;
-
Name of the alternate supplier: PANAXIA PHARMACEUTICAL INDUSTRIES in partnership with the pharmaceutical establishment operating NEURAXPHARM FRANCE.
Article 2:
This decision is published on the website of the National Agency for the Safety of Medicines and Health Products.
Done on January 25, 2021
Dr Christelle Ratignier Carbonneil
Director General of ANSM
Cannabis companies from Australia, Canada, Israel and the United Kingdom – in partnership with French pharmaceutical distributors – will provide the products for up to 3,000 patients and will not receive any money for supplying the medical cannabis.
French authorities selected main suppliers, as well as substitute suppliers to cover any shortfalls.
The list of suppliers includes:
- Australia-based Althea (one lot as a substitute) and Little Green Pharma (two lots as a main supplier and one lot as a substitute).
- Canada-based Aurora Cannabis (three lots as a main supplier) and Tilray (two lots as a main supplier and two lots as a substitute).
- Israel-based Panaxia (two lots as a main supplier and two lots as a substitute).
- U.K.-based Emmac Life Sciences (two lots as a substitute).
- Read full story at
France chooses companies to supply free medical cannabis for trial program