Here’s what the website says..
The UK Food Standards Agency (FSA) has rejected a Freedom of Information request submitted by HempToday (HT) regarding companies that currently have products in the agency’s CBD safety review, saying the request would create too much of a burden on its staff.
HT sought documentation submitted by eight companies whose products have reached the second stage of the FSA regime for guaranteeing the safety of new or “novel” foods, as well as those for the top 10 companies by number of product submissions that have reached the first stage.
Products that are in the FSA review, which has been troubled from the start, presumably met initial requirements that allow producers to keep their products on the market pending technical aspects of the review. A key rule requires that those products were on retail shelves before February 12, 2020.
Companies were required to submit receipts, contracts or invoices showing their products were in circulation before the February 2020 cutoff date, but the FSA has no mechanism in place to verify that documentation. Critics have said it’s highly unlikely that all of the 12,000+ products that have passed the preliminary stage of the FSA review meet that requirement.
Two batches sought
HempToday’s request, filed Aug. 19, 2022 under the UK’s Freedom of Information Act 2000, sought certificates of analysis (COAs), information on the production process and testing methodology, and documentation that products were on the market before the February 2020 cut-off date for the following companies and portfolio numbers, which have been validated at the second stage of the FSA safety review:
- Pureis: RP07
- Brains Bioceutical: RP11
- Charlotte’s Web: RP230
- Cannaray & Health in Harmony: RP450
- Health and Happiness UK Ltd & Bod Healthcare Ltd: RP518
- 4MP & CBDex: RP70
- cbdMD: RP793
- CBDex: RP85
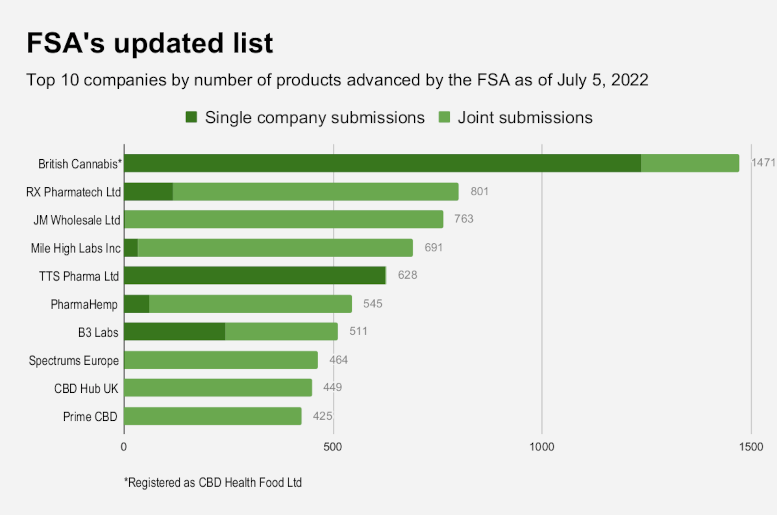
HT also sought documentation proving market-start dates for portfolios of the top 10 companies based on the greatest number of products approved at the first stage of the FSA review:
- British Cannabis (registered as CBD Health Food Ltd.): RP RP 220, RP 251,
RP 252, RP 253, RP 254, RP 255, RP 256, RP 325 - RX Pharmatech Ltd.: RP 427
- JM Wholesale Ltd.: RP 438
- Mile High Labs Inc.: RP 349
- TTS Pharma Ltd: RP 520; RP 521; RP 639; RP 654; RP 749; RP 771; RP 779; RP
853; RP 889; RP 890; RP 921; RP 922; RP 981; RP 982 - PharmaHemp: RP 47; RP 821
- B3 Labs: RP 106; RP 349
- Spectrums Europe: RP 46; RP 47; RP 821
- CBD Hub UK: RP 427
- Prime CBD: RP 821
Reason for refusal
Responding to HT’s request, Hameera Chaudhry-Khan, Senior Freedom of Information Advisor on the agency’s Knowledge Information Management and Security Team, said tracking down documentation regarding sale-start dates for the products “would require the FSA to trawl through a large number of files to identify the information you have requested. The request, [without further clarification,] is certain to exceed the appropriate cost limit (£600/$640) and therefore would be refused on grounds of excessive cost.”
Chaudhry-Khan also asked for more details regarding information requested about COAs, processing details and testing.
Caving in to threats
FSA was inundated with CBD applications earlier this year after stakeholders questioned the review process, with some threatening legal action at being excluded. Some of those companies had seen their products rejected while others simply failed to submit applications by the original deadline, March 31, 2021.
The threats and complaints prompted FSA to reopen the process one full year later, which brought a flood of applications that nearly quadrupled the original number of products under review from 3,536 to 12,118. FSA first added 2,445 products, then doubled the list again in a subsequent revision.
Struggling UK and international CBD makers are clamoring to get into the UK market, expected to reach $1 billion over the next few years, amid a massive global crash in the sector that has many of them on the ropes. Analysts have said the UK is now the second biggest CBD market in the world behind the United States.
Safety warnings
FSA is meanwhile urging caution on the part of the buying public. In a statement earlier this year, the agency suggested “consumers should continue to think carefully before consuming CBD products because we don’t know a lot about them.” FSA said women who are pregnant, breastfeeding or taking medication should not consume CBD products, and urged consumers to take no more than 70mg of CBD per day (about 28 drops of 5% CBD) unless a doctor recommends otherwise.
Reflecting the threat to public safety, FSA has also said it took notice of recent lab reports that showed some CBD products on the market contain presumably higher levels of THC than allowed.
HT has asked FSA for a consultation and intends to resubmit a slimmed-down FOI request.
https://hemptoday.net/uk-food-agency-rejects-hemptoday-request-for-documentation-on-cbd-makers/


















